Hospital
Drlogy Hospital Software
- HIMS
- Practice Management Software
- OPD Software
- IPD Software
- ICU Software
- OT Software
- Pharmacy Software
- Hospital Lab Software
- Hospital Radiology Software
- Hospital Billing Software
- MRD Software
- EMR Software
- EHR Software
- Ambulance Management Software
- BioMedical Waste Management Software
- Hospital Software Brochure
Hospital Features
- HIMS Features
- OPD Software Features
- IPD Software Features
- ICU Software Features
- OT Software Features
- EHR Software Features
- EMR Software Features
- MRD Software Features
- Hospital Billing Software Features
- Hospital Lab Software Features
- Hospital Radiology Software Features
- Patient Portal Software Features
- Eye Hospital Software Features
- Dental Hospital Software Features
- Hospital Administration Software Features
Drlogy Specialty Software
- Clinic Software
- Dental Software
- Ophthalmology Software
- Gynecologist Software
- Cardiologist Software
- Urologist Software
- Oncologist Software
- Dermatologist Software
- Gastroenterologist Software
- Pediatrician Software
- Orthopedist Software
- Diabetologist Software
- Homeopathy Software
- General Physician Software
- Ayurvedic Software
- Nephrologist Software
- Rheumatologist Software
- Physiotherapist Software
- Andrologist Software
- ENT Software
- Neurologist Software
- Pulmonologist Software
- IVF Fertility Clinic Software
- Cosmetologist Software
- Endocrinologist Software
- Geriatric Software
- Psychiatrist Software
Hospital Marketing
Hospital Tools
Drlogy ICD Codes
Hospital Software
- Hospital Software in India
- Hospital Software in Mumbai
- Hospital Software in Delhi
- Hospital Software in Bangalore
- Hospital Software in Hyderabad
- Hospital Software in Chennai
- Hospital Software in Pune
- Hospital Software in Kolkata
- Hospital Software in Noida
- Hospital Software in Indore
- Hospital Software in Jaipur
- Hospital Software in Chandigarh
- Hospital Software in Ahmedabad
- Hospital Software in Gurugram
- Hospital Software in Lucknow
Clinic
Drlogy Clinic Software
Clinic Management Tools
Clinic Software Features
- Medical Clinic Software Features
- Dental Software Features
- Ophthalmology Software Features
- Practice Management Software Features
- Orthodontic Software Features
- Eye Clinic Management Software Features
- Dental EMR Software Features
- Dental Practice Management Software
- Ophthalmology EMR Software Features
- Nursing Home Software Features
Clinic Marketing
Clinic Software
- Clinic Software in India
- Clinic Software in Mumbai
- Clinic Software in Delhi
- Clinic Software in Bangalore
- Clinic Software in Hyderabad
- Clinic Software in Chennai
- Clinic Software in Pune
- Clinic Software in Kolkata
- Clinic Software in Noida
- Clinic Software in Indore
- Clinic Software in Jaipur
- Clinic Software in Chandigarh
- Clinic Software in Ahmedabad
- Clinic Software in Gurugram
- Clinic Software in Lucknow
Pathology Lab
Drlogy Pathology Lab Software
Lab Management Tools
Lab Software Features
Lab Marketing
Lab Equipment Guide
Lab Report Format
Pathology Lab Software
- Pathology Lab Software in India
- Pathology Lab Software in Mumbai
- Pathology Lab Software in Delhi
- Pathology Lab Software in Bangalore
- Pathology Lab Software in Hyderabad
- Pathology Lab Software in Chennai
- Pathology Lab Software in Pune
- Pathology Lab Software in Kolkata
- Pathology Lab Software in Noida
- Pathology Lab Software in Indore
- Pathology Lab Software in Jaipur
- Pathology Lab Software in Chandigarh
- Pathology Lab Software in Ahmedabad
- Pathology Lab Software in Gurugram
- Pathology Lab Software in Lucknow
Report Format
Hematology Report Format
- Blood Report Format
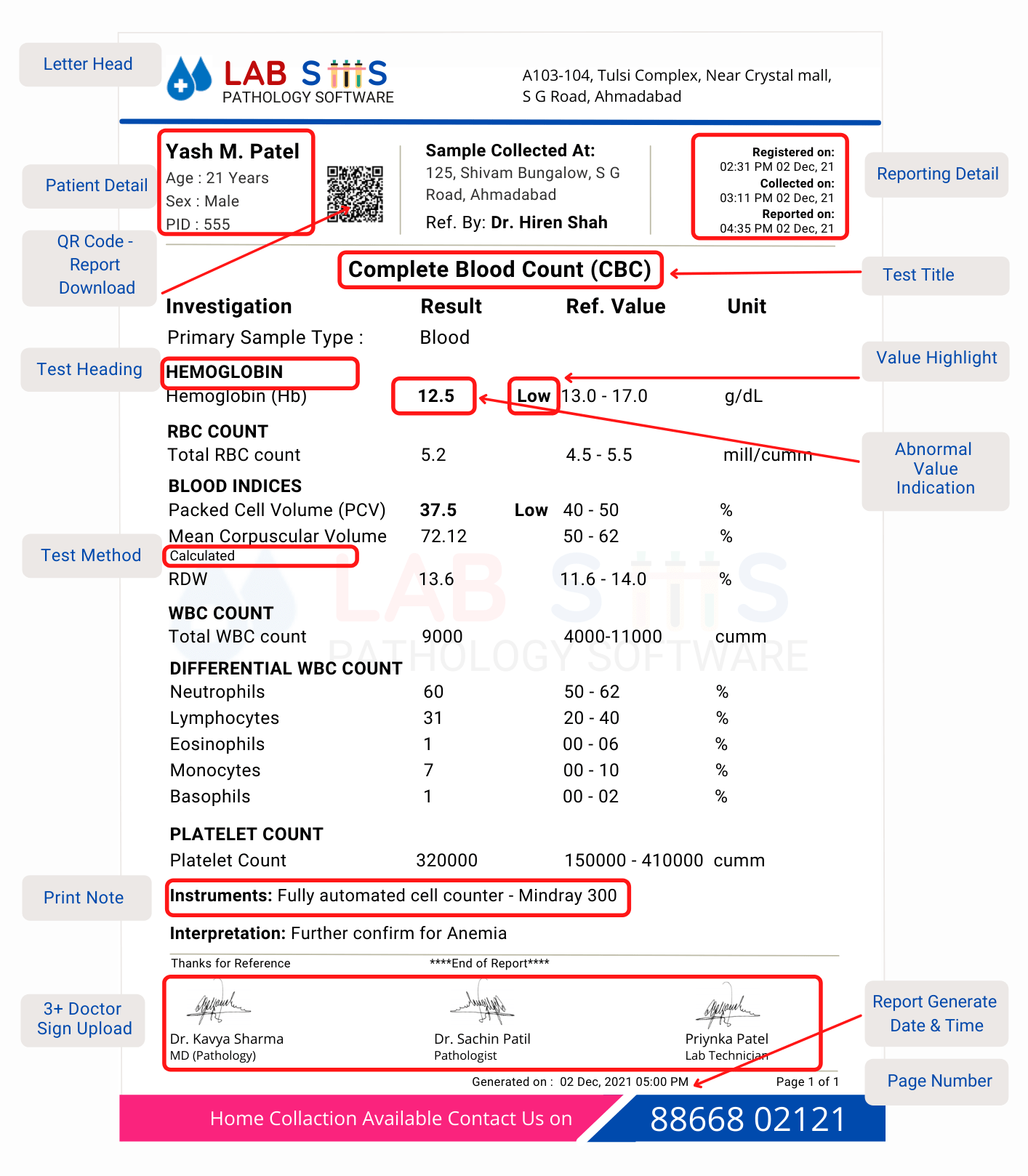
- CBC Report Format
- RBC Count Report Format
- Platelet Count Test Report Format
- WBC Blood Test Report Format
- AEC Report Format
- ALC Test Report Format
- APC Test Report Format
- MCHC Blood Test Report Format
- MCH Blood Test Report Format
- MCV Blood Test Report Format
- MPV Blood Test Report Format
- HCT Blood Test Report Format
- PDW Blood Test Report Format
- ESR Report Format
- ABC Test Report Format
- AMC Test Report Format
- Hemoglobin Test Report Format
- DLC Test Report Format
- APTT Test Report Format
- Prothrombin Time Report Format
- Reticulocyte Count Report Format
- Fibrinogen Test Report Format
- Direct Coombs Test Report Format
- Indirect Coombs Report Format
- Prothrombin Time Report Format
- Clotting Time Test Report Format
- Peripheral Blood Report Format
- Factor VIII Test Report Format
- Factor XII Test Report Format
- Factor XIII Test Report Format
- Coagulation Profile Report Format
- Bleeding Time Test Report Format
- DLC Test Report Format
- ANC Test Report Format
- AEC Report Format
- Blood Group Test Report Format
- D Dimer Test Report Format
- Sickle Cell Test Report Format
- Factor VII Test Report Format
- Factor XI Deficiency Report Format
- Factor X Test Report Format
- Factor IX Test Report Format
- Factor V Test Report Format
- IPF Test Report Format
Molecular Genetics Format
Biochemisty Report Format
- KFT Test Report Format
- LFT Test Report Format
- Lipid Profile Test Report Format
- Hba1C Test Report Format
- Fasting Blood Sugar Report Format
- Random Blood Sugar Report Format
- Vitamin B12 Report Format
- Vitamin C Test Report Format
- Vitamin D Test Report Format
- Vitamin K Test Report Format
- Vitamin B9 Test Report Format
- Vitamin E Test Report Format
- Indirect Bilirubin Report Format
- HDL Cholesterol Test Report Format
- LDL Cholesterol Test Report Format
- VLDL Cholesterol Report Format
- C Peptide Test Report Format
- Ferritin Test Report Format
- Serum Calcium Test Report Format
- Serum Potassium Report Format
- Serum Electrolytes Report Format
- CMP Blood Test Report Format
- Bun Test Report Format
- Albumin Test Report Format
- Globulin Test Report Format
- AST Test Report Format
- Magnesium Test Report Format
- Lactic Acid Test Report Format
- Serum Iron Test Report Format
- Sodium Blood Test Report Format
- Chloride Blood Report Format
- GGT Blood Test Report Format
- Amylase Test Report Format
- Lipase Test Report Format
- ALP Test Report Format
- ALT Test Report Format
- Ionized Calcium Test Report Format
- Creatinine Test Report Format
- Uric Acid Test Report Format
- Beta Hydroxybutyrate Format
- Phenobarbital Test Report Format
- Flecainide Test Report Format
- Creatinine 24Hr Urine Report Format
- Serum Osmolality Report Format
- TIBC Test Report Format
- Selenium Test Report Format
- Manganese Blood Report Format
- Arterial Blood Gas Report Format
Biochemisty Report Format
- Digoxin Test Report Format
- Serum Iron Test Report Format
- Uric Acid Test Report Format
- FPG Test Report Format
- BNP Test Report Format
- ALT Test Report Format
- B2M Test Report Format
- Creatine Kinase Test Report Format
- Potassium Blood Report Format
- PPBS Test Report Format
- UPCR Test Report Format
- Aldolase Test Report Format
- GSBV Test Report Format
- Haptoglobin Test Report Format
- Prealbumin Blood Report Format
- DNPH Test Report Format
- Sodium Blood Test Report Format
- Phosphorus Blood Report Format
- Chloride Blood Report Format
- acrolimus Test Report Format
- CRT Test Report Format
- ALP Test Report Format
- Creatinine Test Report Format
Microbiology Test Report Format
- Malaria Report Format
- Urine Culture Report Format
- Stool Culture Test Report Format
- Sputum AFB Test Report Format
- KOH Test Report Format
- Group B Strep Test Report Format
- Ova And Parasite Report Format
- Myco Panel Test Report Format
- Gram Stain Test Report Format
- C Difficile Toxin Test Report Format
- RSV Test Report Format
- Pertussis Test Report Format
- Gonorrhea Test Report Format
- Mycobacterial Report Format
- Sputum AFB Test Report Format
Tumor Markers Report Format
- Troponin T Test Report Format
- Troponin I Test Report Format
- CA 125 Test Report Format
- CA 15 3 Test Report Format
- CA 19 9 Test Report Format
- AFP Test Report Format
- DCP Test Report Format
- HER2 Test Report Format
- Galectin 3 Test Report Format
- Pax8 Test Report Format
- Double Marker Test Report Format
- Triple Marker Test Report Format
Immunology Report Format
- CRP Test Report Format
- Widal Test Report Format
- RAST Test Report Format
- Dengue Igm Test Report Format
- Dengue Igg Test Report Format
- Dengue Ns1 Antigen Report Format
- Hepatitis C Virus Report Format
- Hepatitis A Igm Report Format
- Hepatitis A Igg Report Format
- VDRL Test Report Format
- Typhidot Test Report Format
- HIV Test Report Format
- Mantoux Test Report Format
- Hepatitis B Profile Report Format
- Hepatitis B Core Report Format
- Hepatitis B Surface Report Format
- HBSAG Quantitative Report Format
- IGA Test Report Format
- IGM Test Report Format
- IGE Test Report Format
- IGG Test Report Format
- Anti CCP Test Report Format
- Hepatitis B Surface Report Format
- G6PD Test Report Format
- Dengue Test Report Format
- Dust Allergy Test Report Format
- Anti B Titre Test Report Format
- Anti A Titre Test Report Format
- Ribosomal P Report Format
- Anti Histone Report Format
- IGA Test Report Format
- IGM Test Report Format
- IGE Test Report Format
- IGG Test Report Format
- GQ1B Antibody Igg Report Format
- GM1 Antibody Igg Report Format
- GM1 Antibody Igm Report Format
- GD1B Antibody Igg Report Format
- GD1A Antibody Igm Report Format
- GD1A Antibody Igg Report Format
- SLA Test Report Format
- GAD 65 Test Report Format
- TNF Alpha Test Report Format
- Torch Profile Test Report Format
- Arthritis Profile Test Report Format
- HS CRP Test Report Format
- ASO Test Report Format
- Rheumatoid Factor Report Format
- Toxoplasmosis Test Report Format
- B2GPI Test Report Format
Clinical Pathology Report Format
Histopathology Report Format
Endocrinology Report Format
- TSH Test Report Format
- Thyroid Antibodies Report Format
- Thyroid Panel Test Report Format
- Prolactin Test Report Format
- Beta Hcg Test Report Format
- Cortisol Test Report Format
- Progesterone Test Report Format
- Testosterone Test Report Format
- T3 Test Report Format
- T4 Test Report Format
- FSH Test Report Format
- LH Test Report Format
- Estradiol Test Report Format
- DHEA Test Report Format
- DHEA Sulfate Test Report Format
- Papp A Test Report Format
- Inhibin B Test Report Format
- Inhibin A Test Report Format
- Calcitonin Test Report Format
Cytopathology Report Format
Radiollogy Report Format
Radiology
Drlogy Radiology Software
Radiology Management Tools
Radiology Report Format
Radiology Software Features
- RIS Features
- X Ray Reporting Software Features
- Sonography Software Features
- CT Scan Software Features
- MRI Software Features
- Radiology Reporting Software Features
- Medical Imaging Software Features
- Sonography Reporting Software Features
- Radiology Imaging Software Features
- X Ray Imaging Software Features
- USG Reporting Software Features
Radiology Marketing
Radiology Software
- Radiology Software in India
- Radiology Software in Mumbai
- Radiology Software in Delhi
- Radiology Software in Bangalore
- Radiology Software in Hyderabad
- Radiology Software in Chennai
- Radiology Software in Pune
- Radiology Software in Kolkata
- Radiology Software in Noida
- Radiology Software in Indore
- Radiology Software in Jaipur
- Radiology Software in Chandigarh
- Radiology Software in Ahmedabad
- Radiology Software in Gurugram
- Radiology Software in Lucknow
 Drlogy
Drlogy