Drlogy
Healthcare organization
UPCR Test Report Format: 10 Key Clinical Guidelines & Example
In the realm of medical diagnostics, the Urine Protein to Creatinine Ratio (UPCR) test holds significant importance for assessing kidney function and detecting potential renal abnormalities. The laboratory report format for the UPCR test is a crucial document that conveys essential information to healthcare providers.
This report sets the stage for the detailed analysis of urine samples, emphasizing its pivotal in diagnosing kidney-related conditions and guiding appropriate medical interventions.
10 Key UPCR Test Report Format Clinical Guidelines
Below are the 10 key clinical guidelines for formatting a UPCR Test report in your pathology laboratory.
1. Patient Information: Name, Age, Gender, etc.
- Include full name, date of birth, gender, and contact details.
- Verify and cross-reference patiententifiers for accuracy.
- Ensure compliance with data protection regulations.
- Collect relevant medical history for comprehensive analysis.
- Communicate effectively with the patient for informed consent.
2. Reference Doctor Information
- Clearly display the referring physician's name, contact information, and medical license.
- Utilize the reference doctor's official letterhead for formal communication.
- Foster a collaborative relationship by acknowledging the referring healthcare professional.
- Provide a dedicated space for additional notes or specific instructions from the referring doctor.
- Maintain confidentiality and adhere to privacy regulations.
3. Specimen Information: Name, Volume & Collection
- Clearly label the specimen with the patient's name and uniqueentifier.
- Document the volume of the collected urine sample in milliliters.
- Specify proper collection techniques to ensure sample integrity.
- Include information on any preservatives or special handling requirements.
- Communicate clear instructions for timely delivery to the laboratory.
4. Test Name Heading, Test Methodology
- Clearly state "UPCR Test" as the heading for easyentification.
- Provide a brief description of the UPCR test methodology.
- Highlight any specific variations or modifications in the testing process.
- Ensure alignment with established medical standards and guidelines.
- Offer educational material to enhance patient understanding of the test.
5. Test Result
- Present UPCR test results in a clear and concise format.
- Include units of measurement (mg/mg) for accurate interpretation.
- Highlight abnormal values with appropriate symbols or color-coding.
- Specify any comments or additional information related to the test results.
- Provide information on the significance of the results for the referring physician.
6. Normal Value Reference
- Clearly state the normal reference range for UPCR (e.g., < 0.2 mg/mg).
- Emphasize the importance of comparing individual results to the established norm.
- Highlight deviations from the normal range for prompt attention.
- Include age-specific or demographic considerations when applicable.
- Facilitate better patient and clinician understanding of result interpretation.
7. Interpretation & Instrumentation
- Offer a succinct interpretation of UPCR results, indicating normal or abnormal findings.
- Specify the specific instrumentation and technology employed in the analysis.
- Address potential limitations or factors that may affect result interpretation.
- Encourage consultation with a healthcare professional for result discussion and further action.
- Provide references to relevant clinical guidelines for additional context.
8. Signature and Date
- Mandate a signature from the authorized laboratory personnel responsible for result validation.
- Clearly display the date of report issuance to ensure timeliness.
- Facilitate traceability and accountability in the reporting process.
- Include the credentials of the signing laboratory professional.
- Confirm the authenticity of the report and the responsible personnel.
9. QR Code Authenticity and Barcode
- Implement QR codes for secure result verification and access to additional information.
- Utilize barcodes to streamline tracking and record-keeping processes.
- Enhance data security by linking the QR code to a secure online portal for result retrieval.
- Ensure compatibility with commonly used QR code scanners and barcode readers.
- Communicate the purpose of the QR code and barcode for patient and clinician understanding.
10. Diagnostic Laboratory Details
- Clearly present the diagnostic laboratory's name, address, and contact details.
- Include accreditation information and certification details.
- Display any relevant affiliations with professional organizations.
- Convey a commitment to quality and adherence to industry standards.
- Provide information on the laboratory's areas of specialization, if applicable.
Also Check
Drlogy Plus For Complete Digital Solutions for Doctors, Clinics, Hospitals & Labs to Enhance Patient Experience
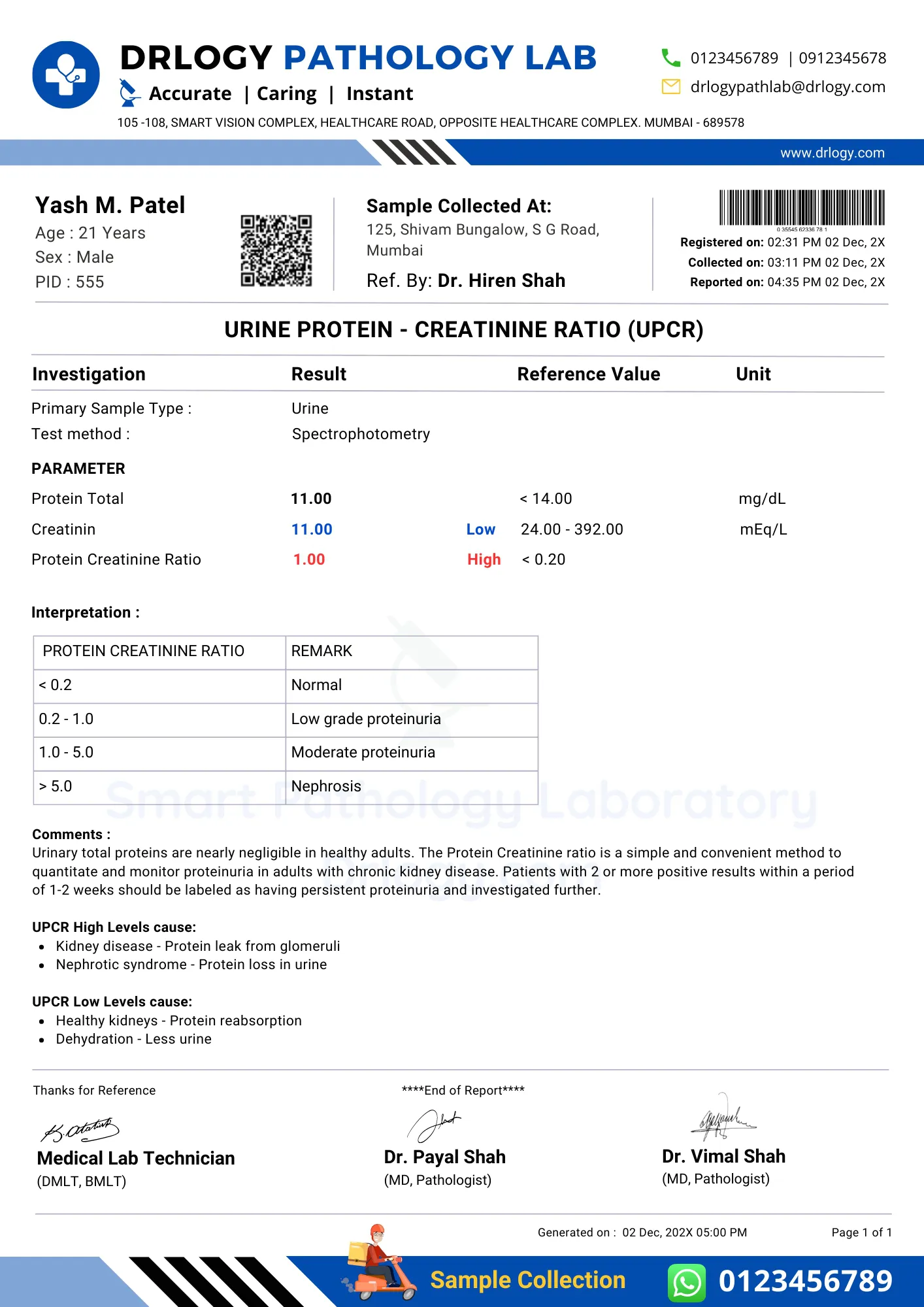
UPCR Test Report Format Sample
UPCR Test Report Format
Here is a UPCR test report PDF format, highlighting its significance in the pathology laboratory.
Drlogy Pathology lab software plays a pivotal in ensuring a UPCR Test Report Format. Additionally, Pathology lab software automates many aspects of the testing process, from sample handling to data analysis. Drlogy Pathology Software provides healthcare providers with real-time access to UPCR test results, enabling timely decision-making and faster patient care.
Referred
Conclusion
- In conclusion, the meticulous adherence to these clinical guidelines for the UPCR test report format is paramount in ensuring accurate diagnosis and effective patient care.
- A comprehensive and well-structured report facilitates precise interpretation by healthcare professionals and fosters transparency, thereby playing a crucial in the early detection and management of kidney-related conditions.
- Drlogy Plus For Complete Digital Solutions for Doctors, Clinics, Hospitals & Labs to Enhance Patient Experience.
Reference