Drlogy
Healthcare organization
TPMT Test Report Format: 10 Key Clinical Guidelines & Sample PDF Example
The TPMT Test Lab Report Format is essential for medical laboratories to convey crucial information about patients' enzyme activity. This standardized format ensures clear communication between healthcare professionals, enabling them to personalize medication dosages effectively.
This streamlined approach enhances patient safety, minimizes adverse reactions, and optimizes therapeutic outcomes, highlighting the significance of accurate and comprehensible lab reports in clinical practice.
Recommended
10 Key TPMT Test Report Format Clinical Guidelines
Below are the 10 key clinical guidelines for formatting a TPMT Test report in your pathology laboratory.
1. Patient and Doctor Details:
- Patient's name, age, gender, contact information, and uniqueentifier.
- Reference doctor's name, contact details, medical license number, and specialty.
- Use official letterhead with the diagnostic laboratory's logo, address, and contact information.
- Ensure patient confidentiality and compliance with data protection regulations.
- Include any relevant clinical history or additional information provided by the referring physician.
2. Specimen Information:
- Clearly state the specimen type (e.g., blood, saliva), volume collected, and collection date/time.
- Specify the test name (TPMT Test) and the methodology used (e.g., PCR, enzyme activity assay).
- Ensure proper specimen labeling and handling to prevent errors or contamination.
- Include information on any specific requirements for sample collection, storage, or transport.
- Verify that the specimen received matches the information provided on the requisition form.
3. Test Result and Normal Values:
- Present the TPMT test result in numerical values with the corresponding units of measurement.
- Provide the reference range for normal TPMT enzyme activity levels (e.g., 24.0 - 44.0 U/mL).
- Highlight any deviations from the normal range that may require clinical attention.
- Include interpretation guidelines to help clinicians understand the significance of the test result.
- Emphasize the importance of considering clinical context when interpreting results.
4. Quality Control:
- Describe the quality control measures implemented during the testing process.
- Include details of internal quality control procedures, such as calibration and validation.
- Highlight adherence to relevant accreditation standards and regulatory requirements.
- Ensure that all equipment used for testing is properly maintained and calibrated.
- Document any deviations from expected results and corrective actions taken.
5. Interpretation and Instrumentation:
- Provide guidance on interpreting TPMT test results based on the patient's genotype and clinical context.
- Explain the significance of TPMT enzyme activity levels in relation to thiopurine drug metabolism.
- Describe the instrumentation and methodology used for TPMT testing (e.g., spectrophotometry, genetic sequencing).
- Discuss the implications of TPMT variants on drug dosing and treatment outcomes.
- Collaborate with clinical experts to develop standardized interpretation guidelines.
6. Signature, Date, and Authenticity:
- Obtain a signature from the authorized laboratory personnel who performed or reviewed the test.
- Include the date of the report to indicate the timeliness of the results.
- Implement QR code authenticity and barcode systems to enhance report traceability and security.
- Ensure that all signatures are legible and accompanied by the signer's credentials.
- Verify the accuracy of patient and test information before finalizing the report.
7. Diagnostic Laboratory Information:
- Provide comprehensive details about the diagnostic laboratory, including its name, accreditation status, and certification.
- Include contact information for the laboratoryector or supervisor responsible for overseeing testing procedures.
- Specify the laboratory's operating hours, specimen collection requirements, and turnaround time for test results.
- Ensure compliance with applicable regulatory requirements and standards for medical laboratories.
- Offer resources for patients and healthcare providers to access additional information or support services.
8. Additional Notes and Disclaimers:
- Include any additional information or clarifications relevant to the TPMT test or its interpretation.
- Address common questions or concerns that may arise regarding the test procedure or results.
- Provide disclaimers regarding the limitations of the TPMT test, including factors that may influence its accuracy or reliability.
- Offer guidance on follow-up testing or alternative diagnostic approaches if needed.
- Encourage open communication between healthcare providers and the diagnostic laboratory to address any uncertainties or discrepancies.
9. Reporting Format Consistency:
- Ensure consistency in formatting and layout throughout the TPMT test report for clarity and professionalism.
- Use standardized terminology and abbreviations to enhance readability and comprehension.
- Organize information logically, with clear headings and subheadings to facilitate navigation.
- Review the report template regularly to incorporate any updates or improvements based on feedback or new developments.
- Seek input from stakeholders, including healthcare providers and patients, to ensure that the report format meets their needs and preferences.
10. Compliance with Regulatory Standards:
- Adhere to relevant regulatory standards and guidelines governing medical laboratory reporting, including HIPAA, CLIA, and ISO 15189.
- Stay informed about changes or updates to regulations and adjust reporting practices accordingly.
- Maintain documentation of compliance activities, including audits, inspections, and proficiency testing results.
- Participate in external quality assessment programs to benchmark performance andentify areas for improvement.
- Collaborate with regulatory agencies, professional organizations, and industry partners to promote best practices in laboratory reporting and quality assurance.
Also Check
Drlogy Plus For Complete Digital Solutions for Doctors, Clinics, Hospitals & Labs to Enhance Patient Experience
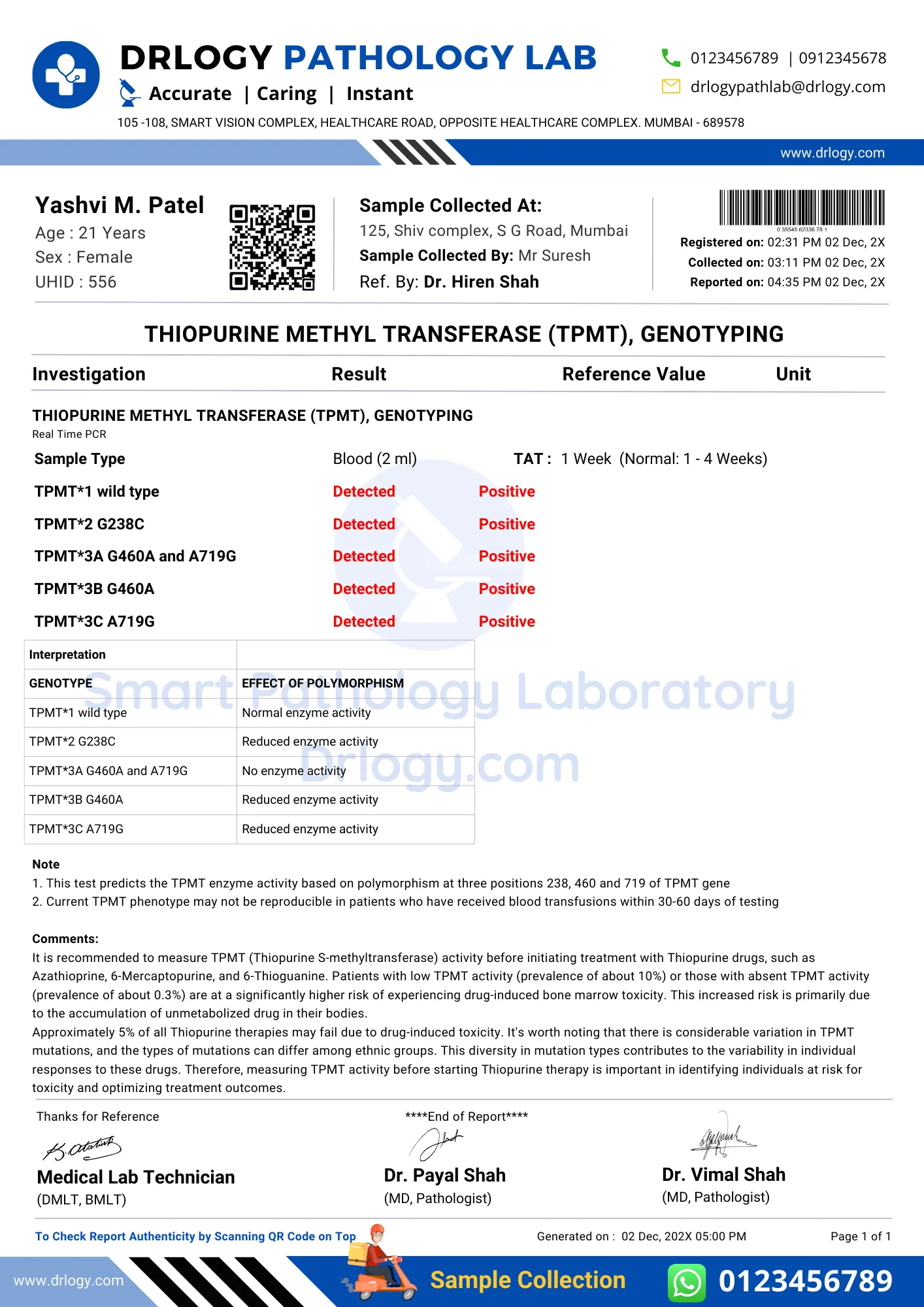
TPMT Test Positive Report Format Sample
TPMT Test Negative Report Format Sample

TPMT Test Report Format PDF
Here is a TPMT test report PDF format, highlighting its significance in the pathology laboratory.
Drlogy Pathology lab software plays a pivotal in ensuring a TPMT Test Report Format. Additionally, Pathology lab software automates many aspects of the testing process, from sample handling to data analysis. Drlogy Pathology Software provides healthcare providers with real-time access to TPMT test results, enabling timely decision-making and faster patient care.
Referred
Conclusion
- In conclusion, the TPMT test report format plays a critical in ensuring accurate interpretation and effective communication of patient results.
- Consistency in reporting format, adherence to quality control measures, and compliance with regulatory standards underscore the importance of reliable and trustworthy laboratory reports in optimizing patient care and safety.
- Drlogy Plus For Complete Digital Solutions for Doctors, Clinics, Hospitals & Labs to Enhance Patient Experience.
Reference