Drlogy
Healthcare organization
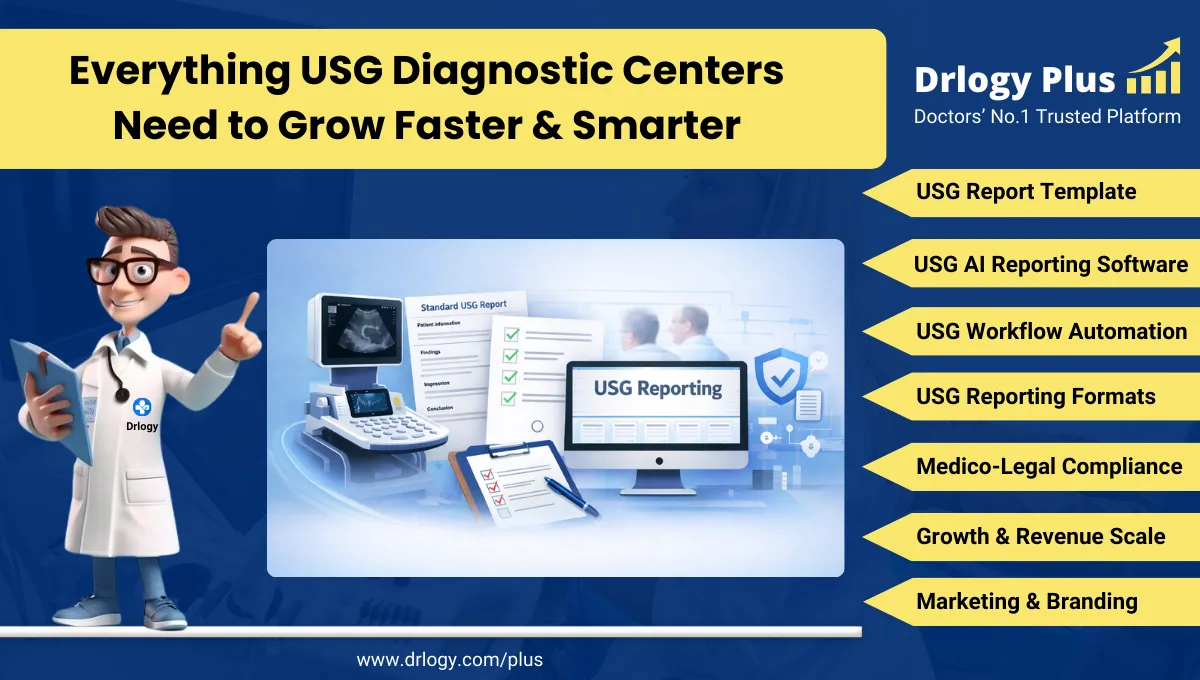
Malaria Report Format: 10 Key Guidelines, PDF & Example
The malaria test report holds paramount significance within the domain of pathology laboratories. Malaria, a widespread parasitic disease caused by Plasmodium species, particularly prevalent in tropical and subtropical regions, necessitates accurate diagnosis for prompt and appropriate patient management.
The malaria report not only aids in confirming a malaria infection but also guides clinicians in selecting the most effective treatment, thereby enhancing patient care and helping to control the spread of the disease. Ensuring a standardized and comprehensive format for the malaria test report is essential for clear and concise communication of results to both healthcare professionals and patients.
Recommended
10 Key Malaria Report Format Clinical Guidelines
Below are the 10 key clinical guidelines for formatting a Malaria Test Report in your pathology laboratory.
1. Patient Information:
- Full name and age of the patient
- Date of sample collection
- Unique patiententifier (ID)
- Relevant medical history
- Referring physician details
2. Sample Details:
- Sample type (e.g., blood smear, rapid diagnostic test)
- Date and time of sample receipt
- Source of the sample (e.g., venous blood, capillary blood)
- Pre-analytical handling details
- Sample condition upon receipt (e.g., hemolyzed, clotted)
3. Test Methodology:
- Description of the test performed (e.g., microscopy, PCR)
- Testing laboratory's name and accreditation information
- Details about staining techniques used
- Instrumentation or equipment employed in testing
- Quality control measures implemented
4. Parasiteentification:
- Presence or absence of malarial parasites
- Speciesentification (Plasmodium falciparum, P. vivax, etc.)
- Parasite count per microliter of blood
- Morphological characteristics ofentified parasites
- Staging of parasites (ring forms, trophozoites, schizonts, etc.)
5. Results Interpretation:
- Interpretation of test results (positive, negative, inconclusive)
- Comments on the clinical significance of the findings
- Recommendations for further diagnostic or confirmatory testing
- Consideration of patient's symptoms and travel history
- Drug susceptibility/resistance information if applicable
6. Quality Assurance and Controls:
- Details about internal and external quality controls
- Mention of proficiency testing participation and outcomes
- Verification of test accuracy and precision
- Compliance with relevant standards and guidelines
- Actions taken in case of quality control deviations
7. Turnaround Time and Reporting:
- Total time taken for the test, from sample receipt to reporting
- Explanation of any delays and reasons for extended TAT
- Definition of urgent or critical TAT for malaria testing
- Process for result communication to the healthcare provider
- Reporting format (electronic, hard copy, etc.)
8. Signature and Credentials:
- Name and designation of the reporting laboratory professional
- The signature or electronic authorization of the reporting individual
- Date and time of report authorization
- Contact information for further inquiries
- Professional certification or accreditation details
9. Patient Counseling and Education:
- Guidance for the patient on malaria prevention and control
- Information on appropriate treatment options
- Instructions on medication adherence and dosage
- Advice on follow-up testing and monitoring
- Contact information for healthcare assistance
10. Confidentiality and Data Security:
- Assurance of patient data confidentiality
- Compliance with relevant data protection regulations
- Measures taken to safeguard patient information
- Access control and restricted data sharing practices
- Disposal procedures for sensitive data and records
Malaria Normal Report Format Sample

Malaria Positive Report Format Sample

Malaria Report Format PDF
Here is a Malaria Test Report in PDF format, highlighting its significance in the pathology laboratory.
Normal Malaria Parasiteentification Test Report Format PDF
Positive Malaria Parasiteentification Test Report Format PDF
Drlogy Pathology lab software plays a pivotal in ensuring a good Malaria Test Report Format. Additionally, Pathology lab software automates many aspects of the testing process, from sample handling to data analysis. Drlogy Pathology Software provides healthcare providers with real-time access to Malaria test results, enabling timely decision-making and faster patient care.
Referred
Conclusion
- A well-structured and standardized malaria report format is imperative for efficient communication and comprehension of the diagnostic findings in the realm of pathology.
- It acts as a vital tool that enables healthcare providers to accurately diagnose malaria infections, select appropriate treatment regimens, and monitor the effectiveness of interventions.
- By adhering to established guidelines and including essential components such as test results, speciesentification, recommendations, and quality control measures, the malaria test report facilitates streamlined patient care and aids in the overall management of this significant public health concern.
- A properly formatted and comprehensive malaria test report ultimately contributes to improved healthcare outcomes and plays a pivotal in the ongoing battle against this global health issue.
Reference
- Malaria - Wikipedia [1].
- Rapid Diagnostic Tests for Malaria Parasites - PMC [2].
- Malaria Parasiteentification Test - Drlogy [3].