Drlogy
Healthcare organization
Cervix Biopsy Test Report Format: 10 Clinical Guidelines & Sample PDF
The cervix biopsy test is an essential diagnostic procedure conducted in medical laboratories to evaluate cervical tissue for abnormalities, such as precancerous changes and cervical cancer.
The resulting lab report provides crucial insights into the cellular characteristics of the tissue, guiding healthcare providers in making accurate diagnoses and formulating effective treatment plans.
Recommended
10 Key Cervix Biopsy Test Report Format Clinical Guidelines
Below are the 10 key clinical guidelines for formatting a Cervix Biopsy Test report in your pathology laboratory.
1. Patient Information:
- Include patient's name, age, gender, and other relevant demographic details.
- Ensure accuracy and clarity in recording patient information.
- Follow privacy and confidentiality protocols for handling patient data.
- Double-check patiententifiers to prevent errors in reporting.
- Provide contact information for further inquiries or updates.
2. Reference Doctor Information:
- Document the referring physician's name, contact details, and specialty.
- Verify the referral source to maintain accountability and traceability.
- Communicate any specific instructions or preferences from the referring doctor.
- Acknowledge the collaboration between the diagnostic lab and the referring physician.
- Establish clear channels for communication and consultation between parties.
3. Specimen Information:
- Clearly label the specimen with its name, volume, and collection details.
- Ensure proper handling and storage of the specimen to maintain integrity.
- Document any deviations or abnormalities in specimen collection or condition.
- Provide instructions for transporting and processing the specimen.
- Verify specimenentification to prevent sample mix-ups or errors.
4. Test Name Heading and Methodology:
- Clearly state the name of the test being performed (Cervix Biopsy Test).
- Describe the methodology used for sample analysis and diagnostic evaluation.
- Provide a brief overview of the test procedure and its clinical significance.
- Include any relevant guidelines or standards followed in conducting the test.
- Ensure consistency in test naming conventions across reports and records.
5. Test Result:
- Present the test result clearly and concisely, indicating normal or abnormal findings.
- Use standardized terminology and units for reporting test results.
- Highlight any significant deviations from normal ranges or expected outcomes.
- Include reference ranges or thresholds for interpretation, if applicable.
- Offer interpretation guidance or clinical context to aid understanding.
6. Quality Control Information:
- Document the quality control measures employed during test analysis.
- Include details of internal and external quality assurance processes.
- Report any quality control failures or deviations and their impact on results.
- Maintain records of instrument calibration, maintenance, and performance checks.
- Ensure compliance with regulatory standards and accreditation requirements.
7. Interpretation and Instrumentation:
- Provide a detailed interpretation of the test results in clinical context.
- Explain the significance of findings and their implications for patient management.
- Clarify any uncertainties or limitations in interpretation due to technical factors.
- Describe the instrumentation used for test analysis and its validation.
- Discuss any correlations with clinical history or other diagnostic tests.
8. Signature and Date:
- Obtain authorized signatures from qualified personnel for report validation.
- Include the date of report issuance to indicate currency and timeliness.
- Ensure compliance with regulatory requirements for signature authentication.
- Maintain records of signatories and their credentials for accountability.
- Implement electronic signature systems for efficient and secure documentation.
9. QR Code Authenticity and Barcode:
- Incorporate QR codes for quick and accurate retrieval of patient and test information.
- Use barcoding systems for specimen tracking,entification, and traceability.
- Ensure compatibility with laboratory information management systems (LIMS).
- Validate QR code authenticity to prevent tampering or unauthorized access.
- Implement data encryption and security protocols for QR code and barcode usage.
10. Diagnostic Laboratory Details:
- Provide comprehensive information about the diagnostic laboratory, including name, address, and contact details.
- Include accreditation status, certifications, and accreditations to demonstrate quality and reliability.
- Specify turnaround times for test processing and reporting.
- Communicate any special instructions or requirements for test requisition and specimen submission.
- Offer assistance and support services for inquiries, consultations, and result interpretation.
Also Check
Drlogy Plus For Complete Digital Solutions for Doctors, Clinics, Hospitals & Labs to Enhance Patient Experience
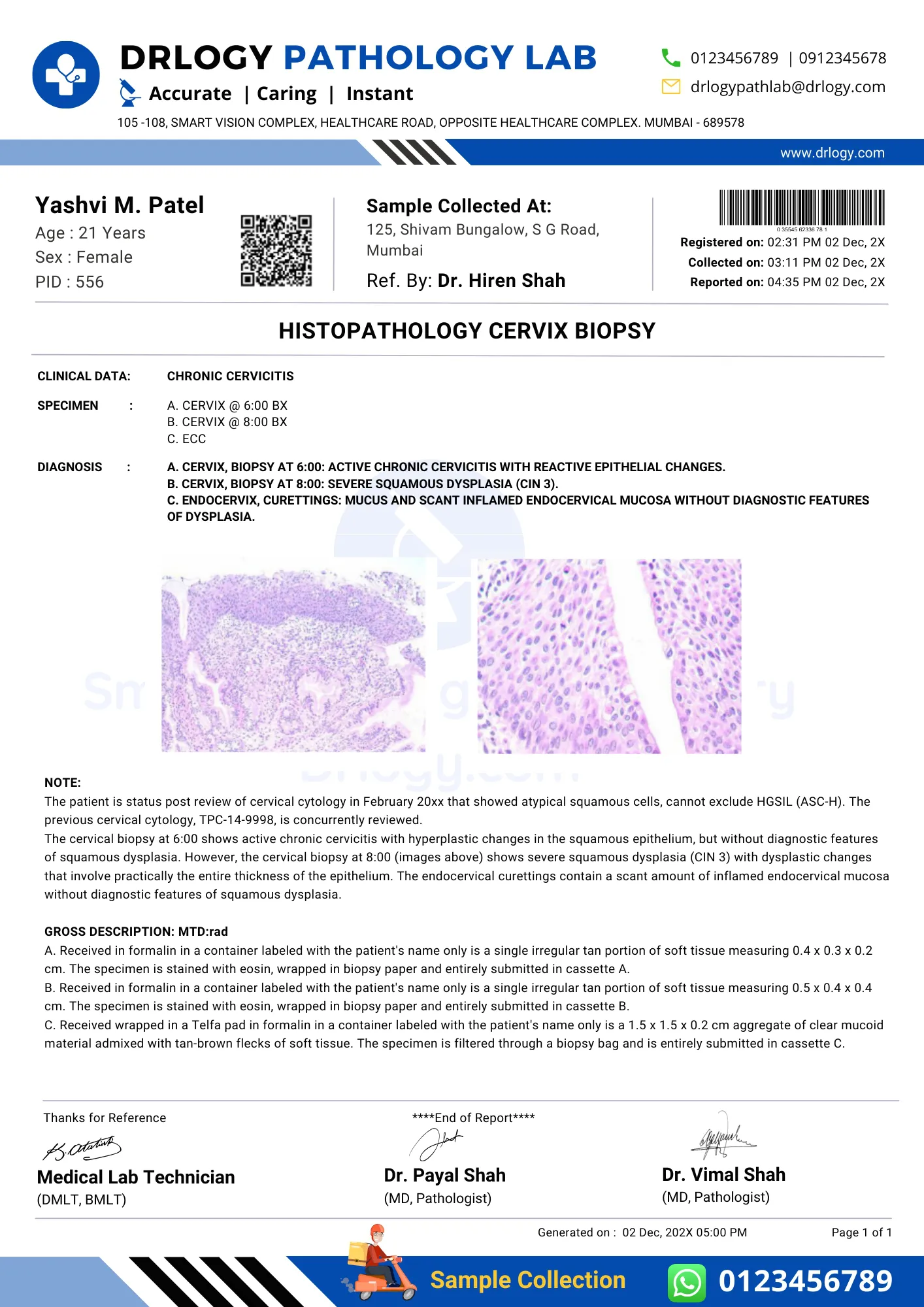
Cervix Biopsy Test Report Format Sample
Cervix Biopsy Test Report Format PDF
Here is a Cervix Biopsy test report PDF format, highlighting its significance in the pathology laboratory.
Drlogy Pathology lab software plays a pivotal in ensuring a Cervix Biopsy Test Report Format. Additionally, Pathology lab software automates many aspects of the testing process, from sample handling to data analysis. Drlogy Pathology Software provides healthcare providers with real-time access to Cervix Biopsy test results, enabling timely decision-making and faster patient care.
Referred
Conclusion
- By adhering to established guidelines and standards, diagnostic laboratories uphold their commitment to delivering reliable and actionable insights crucial for preserving women's reproductive health.
- A well-structured and informative test report not only facilitates precise diagnosis and treatment planning but also promotes patient safety and satisfaction.
- Drlogy Plus For Complete Digital Solutions for Doctors, Clinics, Hospitals & Labs to Enhance Patient Experience.
Reference